New data in AJCN: HiPP COMBIOTIC® shows positive effects on gut microbiota profiles

06.2023
The two studies GOLF I & GOLF II (GO= GOS, LF= L. fermentum) investigated the safety and effects of infant formula with pre- and probiotics.
New data from the GOLF III trial in the American Journal of Clinical Nutrition (AJCN) show: Synbiotic infant formula fortified with GOS and Limosilactobacillus fermentum CECT 5716 leads to similar intestinal parameters as in breastfed infants.1
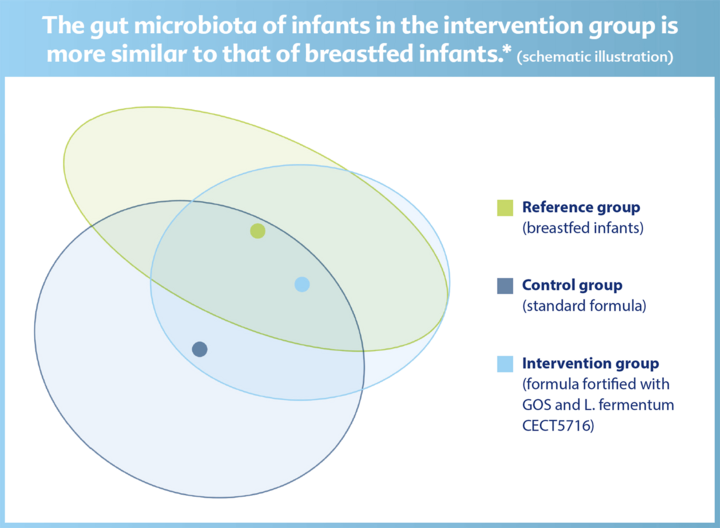
The new GOLF III trial shows:
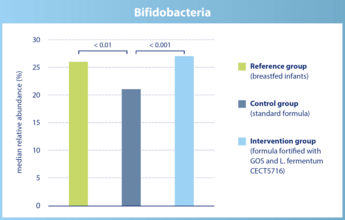
The abundance of bifidobacteria in infants of the intervention group does not differ significantly from breastfed infants.
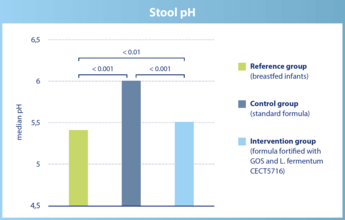
The stool pH in infants of the intervention group is significantly lower than in infants of the control group. It is similar to the stool pH in breastfed infants.
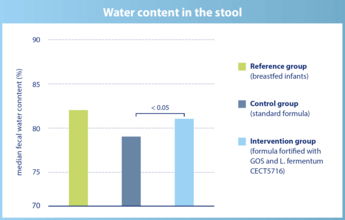
The water content in the stool is significantly higher in infants of the intervention group and comparable to breastfed infants.
Read more
Reference:
(1) Lagkouvardos I et al. Early life gut microbiota profi les linked to synbiotic formula eff ects: a radomized clinical trial in European infants. Am J Clin Nutr 2023; 117 (2): 326–339.
HiPP ORGANIC COMBIOTIC® Products
All product information about our HiPP ORGANIC COMBIOTIC®.



