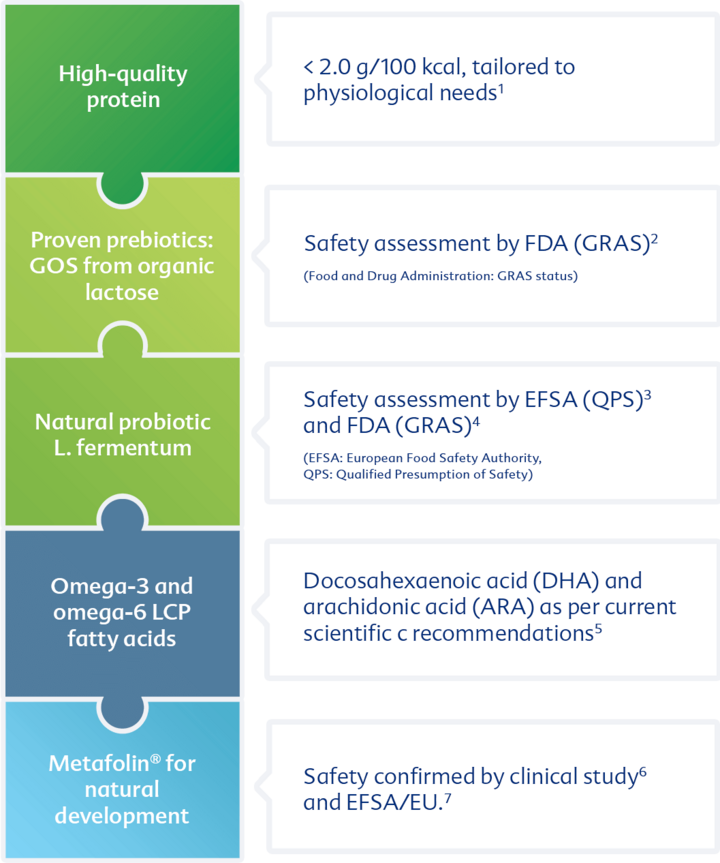
Appropriately low protein content and improved protein quality
Less is more: HiPP 1 COMBIOTIC® has an appropriately low protein content
Breast milk is also considered to be the Gold Standard for protein intake! The appropriately reduced protein intake of infants could provide an effective contribution to preventing obesity in children1. This is why the experts recommend the promotion of breastfeeding and reduction of the protein content in infant formula.
HiPP has put these current recommendations into practice and is now offering HiPP COMBIOTIC® first infant milk with reduced protein content in many countries. Safety, tolerance and the good satiety effect of the new milk have been demonstrated in a clinical study.
Early-protein hypothesis – an explanation
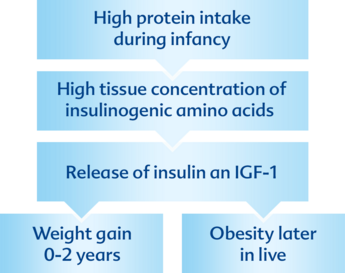
The early-protein hypothesis suggests that high protein intake of infants leads to increased plasma and tissue concentrations of insulin-releasing amino acids through metabolic programming, which in turn stimulates insulin and IGF1 (insulin-like-growth factor 1)8.
Confirmation of the hypothesis through the results of the EU Childhood Obesity Study
The early-protein hypothesis was confirmed by the CHOPs study (EU Childhood Obesity Project) that showed faster weight gain and higher adipogenous activity in the first two years and ultimately a higher long-term risk of adipositas1.
More on the CHOP-Study
References:
1 Koletzko B et al. Am J Clin Nutr 2009; 89(6): 1836-1845
2 FDA. 2008; GRAS Notices GRN No. 236
3 EFSA: The EFSA-Journal 2007; 587: 1-16
4 FDA. 2015; GRAS Notices GRN No. 531
5 Koletzko B et al. J Perinat Med 2008; 36(1): 5-14
6 Troesch B et al. PLoS ONE 2019 14(8): e0216790
7 COMMISSION DELEGATED REGULATION (EU) 2021/571 of 20 January 2021. O cial Journal of the European Union, 8 April 2021. EFSA Journal, doi: 10.2903/j.efsa.2020.5947
8 Koletzko B et al. Am J Clin Nutr 2009; 89: 1502S-1508S
9 Weber M et al. Am J Clin Nutr 2014; 99: 1041-51