Study confirms: HiPP Probiotic is Safe
Natural nourishment from breast milk has been the inspiration for the development of infant formula with pre- and probiotics. In addition to human milk oligosaccharides (HMOs) as prebiotics, breast milk contains a variety of probiotic cultures.
The European Panel of Specialists (ESPGHAN), for example, requires that the safety and benefit of each prebiotic and probiotic substance is established in scientific studies, individually and for any combination of the two. Furthermore, long-term data is required to show what influence the early administration of probiotics may have on later health (1).
L. fermentum and Galactooligosaccharide in infant food
L. fermentum was originally obtained from breast milk and is also a typical coloniser of the infant intestine. It was investigated in numerous cell culture and animal studies, followed by human trials, first in adults and later in infants.
The results indicate that L. fermentum CECT 5716 is not only a safe probiotic strain but also survives the gastrointestinal tract (2, 3) and has a positive influence on the immune system (4, 5, 6, 7).
A randomised, double-blind, controlled study of 121 infants between the ages of 1 and 6 months showed that L. fermentum and GOS are safe in infant formula as well, and also lead to a significant reduction in infections during this time period (8). A study of follow-on formula containing L. fermentum and GOS produced similar results (9).
Follow-up confirms long-term safety
The follow-up to the clinical study of infant formula confirms that formula with GOS and L. fermentum is safe over the long term as well (10). According to the authors, it is the largest long-term study on the safety of probiotics in infant formula.
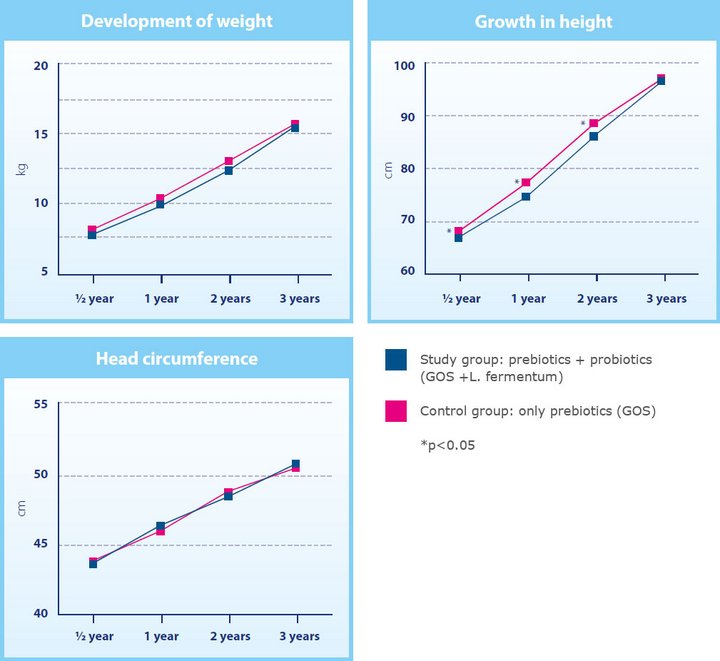
Data from 91 children who had originally participated in the initial 2012 study was used for the randomised follow-up. The primary objective was to record growth by weight, height and head circumference at ages 0.5, 1, 2 and 3 years.
Maldonado et al. showed that the children who received infant formula with GOS and L. fermentum during the first six months of life (the intervention group) had grown adequately in comparison with the children who had received a formula without probiotics (the control group).
At the age of three years, the mean values of weight, height, and head circumference were similar in the children from both groups (figure 1). No differences were found in the incidence of infections, non-infectious diseases and intestinal problems. The intestinal bacteria found in the stool were also comparable in the respective Groups.
Fig. 1 Long-term safety of infant formula (GOLF 2 Follow-up study)10 : Anthropometric data for body weight, body height, head circumference in relation to nutrition
References
1. Braegger C et al. J Pediatr Gastroenterol Nutr 2011; 52: 238–50.
2. Martin R et al. Hum Lact 2005; 21: 8–17.
3. Lara-Villoslada F et al. J Dairy Res. 2009; 76(2): 216–221.
4. Peran L et al. Int J Colorectal Dis 2006; 21(8): 737–746.
5. Olivares M et al. J Appl Microbiol 2006; 101(1): 72–79.
6. Arribas B et al. Br J Nutr 2009; 101(1): 51–8.
7. Peran L et al. Br J Nutr. 2007; 97(1): 96–103.
8. Gil-Campos et al. Pharmacol Res 2012; 65: 231–238.
9. Maldonado et al. J Pediatr 2012; 54: 55–61.
10. Maldonado et al. Pharmacol Res 2015; 95–96: 12–19, epub.


