Current study provides evidence for the allergy-preventive effect of a hydrolysed infant formula
Current study provides evidence for the allergy-preventive effect of a hydrolysed infant formula
Original titel: Freidl R et al. (2023) Extensively Hydrolyzed Hypoallergenic
Infant Formula with Retained T Cell Reactivity. Nutrients 15(1):111.
Background
Cow’s milk allergy (CMA) is one of the most common food allergies in infants and young children. Up to three percent of children are allergic to milk protein, which in the worst case can trigger life-threatening anaphylaxis.
Infant formulae based on hydrolysed (i.e. broken down) cow’s milk protein are a suitable option for allergy risk reduction due to the destruction of IgE epitopes.
In this context, the degree of hydrolysis is crucial, as protein fragments (peptides) that are too long can lead to IgE sensitisation or allergic reactions, while peptides that are too short may fail to induce T-cell tolerance.
Objective of the study
The aim of this in vitro study was to assess an extensively hydrolysed whey-based infant formula (eHF), fortified with galacto-oligosaccharides (GOS) and Limosilactobacillus fermentum CECT 5716 (LF), for its suitability in preventing cow’s milk allergy (CMA). For this purpose, the allergenic potential of the infant formula, as well as its effects on T-cell proliferation and cytokine secretion, were examined.
Study design
Design: In vitro study
Sample materials: Sera from children with diagnosed cow’s milk allergy (n=49) and children without cow’s milk allergy (n=10) as control group
Infant formulae studied: Extensively hydrolysed infant formula (eHF) with GOS, eHF with GOS and LF; infant milk formula based on intact cow’s milk protein (iPF) with GOS; iPF with GOS and LF
Positive control: Pure milk protein
Methods: Immune dot blots, basophil degranulation assays, CFSE dilution assays, xMAP Luminex assays
Results
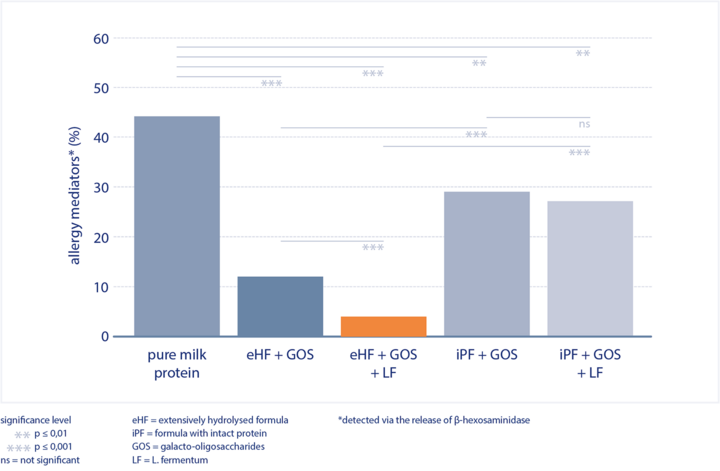
The examined formula based on extensively hydrolysed whey protein with GOS and L. fermentum (eHF + GOS + LF):
- lacks intact cow’s milk allergens, which is why it showed almost no IgE reactivity in the sera of children with CMA (in vitro analysis).
→ no sensitisation of the mast cells - significantly reduced allergenic potential.
→ lowest release of allergy mediators (see figure)
When combined with GOS, eHF significantly reduce the secretion of pro-inflammatory cytokines (IL-17, IL-4, IL-5, IL-13), while maintaining a comparable level of the tolerogenic cytokine IL-10, as compared to formula containing intact cow’s milk protein.
→ possible induction of immunological tolerance
Webinars
Watch the webinar on our website! „New insights in alimentary allergy prevention by improving gut health“ an!
HiPP ORGANIC COMBIOTIC® Products
All product information about our HiPP ORGANIC COMBIOTIC®.
HiPP information material





