Unique combination: reduced protein content, prebiotics, probiotics and ORGANIC quality
- ORGANIC quality for all infant milk formulae1. HiPP is the only producer of infant milk that can draw on more than 20 years of experience in the production of organic infant milk. HiPP organic quality is subject to considerably stricter criteria than those stipulated by law.
- Only lactose: The only sugar in HiPP infant milk formulae is lactose – as in breast milk. Other suppliers add glucose and maltodextrin as additional carbohydrates.
- With LCP: All HiPP infant formulae contain valuable long chain, poly-saturated fatty acids and thus make an essential contribution to good development of the brain, nervous system and eyesight.
- With prebiotics & probiotics: Prebiotic oligosacchardies and probiotic lactic acid cultures are after all, also natural components of breast milk. HiPP milk formula contain the natural probiotic L. fermentum hereditum® that was originally isolated from human milk.
The prebiotics used by HiPP are galacto-oligosaccharides (GOS) that are obtained from lactose and are a natural component of breast milk oligosaccharides.
Notice: Depending on country-specific legislation and recommendation, HiPP COMBIOTIC® formula contain either prebiotics and probiotics or prebiotics alone. - Reduced protein content with improved protein quality: Less is more – HiPP COMBIOTIC® first infant formula - now with reduced protein content. Latest studies have shown that a high protein intake of infants increases the risk of obesity later in life. That is why experts recommend promoting breastfeeding and reducing the protein content of first infant formulae. Read more
- No genetic engineering: HiPP organic baby food does, as a rule, not contain any genetically modified foodstuff, ingredients or feedstuff.
1 Exception: production of HA formulae in organic quality currently impossible for technological reasons.
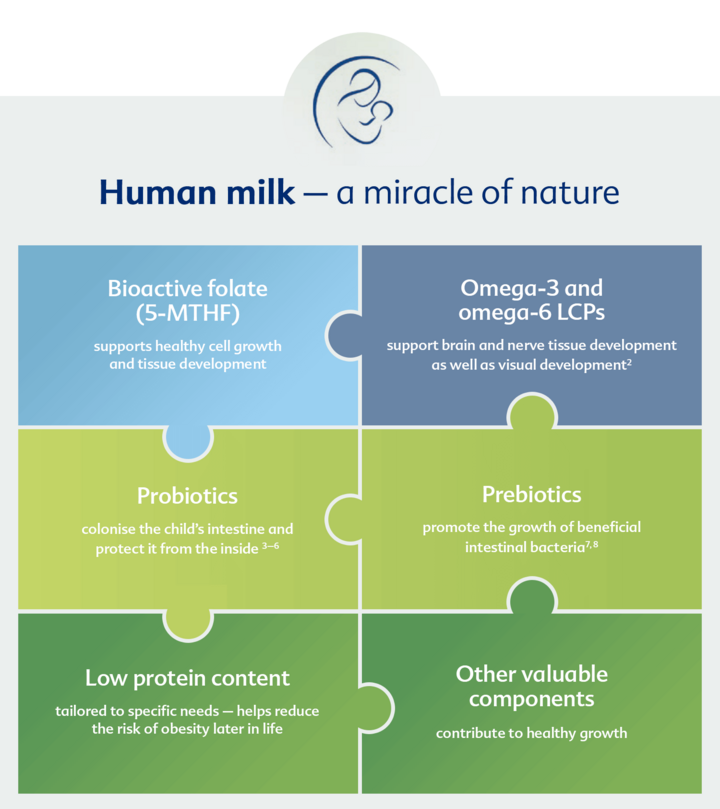
Breast Milk – the Gold Standard
- Its composition is perfectly tailored to infants’ requirements1
- Supplies all the important nutrients for healthy development
- Provides the best possible protection for infants:
- boosts the body’s defences
- protects against gastrointestinal infections
- reduces the risk of illnesses later in life, e.g. obesity and allergies
Breast milk has a wide variety of building blocks that promote an infant’s healthy development in the best possible way.
1 Prell C, Koletzko B. Dtsch Arztebl lnt 2016; 113(25): 435–444.
2 Koletzko B et al AJCN 2020; 111: 10–16.
3 Martin R et al. J Pediatr 2003; 143(6): 754–758.
4 Martin R et al. J Hum Lact 2005; 21(1): 8–17.
5 Gueimonde M et al. Neonatol 2007; 92(1): 64–66.
6 Heikkilä MP, Saris PE. J Appl Microbiol 2003; 95(3): 471–478.
7 Kunz C et al. Annu Rev Nutr. 2000; 20: 699–722.
8 Gibson CR, Roberfroid MB. J Nutr 1995; 125(6): 1401–1412.
What is the difference between the various prebiotics?
The prebiotics of HiPP’s choice are galacto-oligosaccharides (GOS) obtained from lactose. GOS are obtained from lactose (galactose) and are a natural component of breast milk oligo-saccharides.
Benefits of GOS:
- Promoting the formation of a bifidogenic intestinal flora dominated by lactobacilli1-3
- Good tolerability
- Softer stool similar to that of breastfed infants1-4
- Reduction of ph-value in the intestines
- Safe (so called GRAS status by FDA)5
HiPP refuses to use fructo-oligosaccharides (FOS), because FOS are obtained from plants (mainly vegetable such as chicory or onions) and thus not similar to breast milk. Breast milk does not contain any vegetable components!
Critical issues in FOS are in particular the observations of negative impact on the intestinal barrier when FOS is fed to animals: Both the intestinal barrier permeability and salmonella enteritidis translocation were increased, indicating a dysfunctional intestinal barrier and irritation of the intestinal mucosa. These observations in rats were less pronounced in human tests on grown males, but still raise the issue of whether FOS should be used at all on such a vulnerable group as babies whose immune system is not yet fully developed6.
References:
1 Ben XM et al. Chinese Medical Journal 2004; 117(6): 927–931.
2 Fanaro S et al. J Pediatr Gastroenterol Nutr. 2009; 48: 82–88.
3 Sierra C et al. Eur J Nutr 2015; 54(1): 89–99.
4 Ashley C et al. Nutr Journal 2012; 11: 38.
5 FDA. 2008; GRAS Notices GRN No. 236.
6 Ten Bruggencate et al. J Nutr. 2006 Jan;136(1):70-4.


