Clinical study confirms the good tolerability of HiPP COMBIOTIC® infant formulae
Clinical study confirms the good tolerability of HiPP COMBIOTIC® infant formulae
Original titel: Otten et al. (2023) Gastrointestinal Tolerance of an Infant Formula Manufactured from Extensively Hydrolysed Protein in Healthy Term Infants. Nutrients 15, 4674
Original study Otten et al. 2023
Background
Thanks to its composition, human milk is easy to digest and therefore well tolerated. Whenever breastfeeding is not an option, the consensus is to feed infant formula. The ingredients of infant formulae are carefully selected to provide infants with all the essential nutrients. In addition to an optimal nutrient supply, good tolerability is a key requirement for infant formula among parents and healthcare professionals. Functional gastrointestinal disorders (FGIDs), such as colic, regurgitation or constipation, frequently occur with the use of standard infant formulae. Although the symptoms are usually self-limiting and subside with age, FGIDs may be distressing for families and have a massive impact on sleep behaviour, among others. The current market offers a variety of formulae that include ingredients such as pre- and probiotics. It is believed that these functional ingredients have a beneficial impact on the intestinal microbiota, potentially enhancing the tolerability of infant formulae.
Objective of the study
The aim of this analysis was to determine the tolerability of an infant formula based on extensively hydrolysed whey protein in comparison to an infant formula based on intact cow’s milk protein as a control formula using validated parameters during the first 120 days of life.
Study design
Design: prospective, multicentre, randomised, double-blind, controlled intervention study
Study participants: healthy, full-term infants n = 338, of which n = 149 received a formula with extensively hydrolysed protein (eHF) and n = 148 a formula with intact protein as control (CF); the reference group comprised of n = 41 breastfed infants (BF)
Tested infant formulae: eHF with galacto-oligosaccharides (GOS) and Limosilactobacillus fermentum CECT5716 (L. fermentum); CF with GOS and L. fermentum
Methods: 3-day diary, Amsterdam Infant Stool Scale (AISS), Infant Gastrointestinal Symptom Questionnaire (IGSQ)
Results
Results The IGSQ score (lower values indicate higher tolerability) decreased with increasing infant age, did not differ between formula-fed infants (eHF, CF) and was similar to that of breastfed infants (Fig. 1).
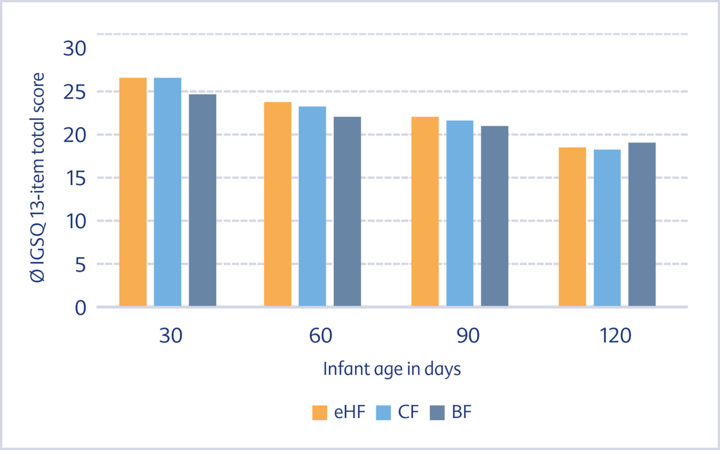
groups during the first four months of life
There were no relevant differences regarding stool frequency and consistency between the eHF and CF groups.
No difference could be observed as regards the sleeping behaviour of the two formula groups throughout the entire observation period. This applies both to the average sleep duration over 24 hours (Fig. 2) and to the average nocturnal sleep duration (Fig. 3).
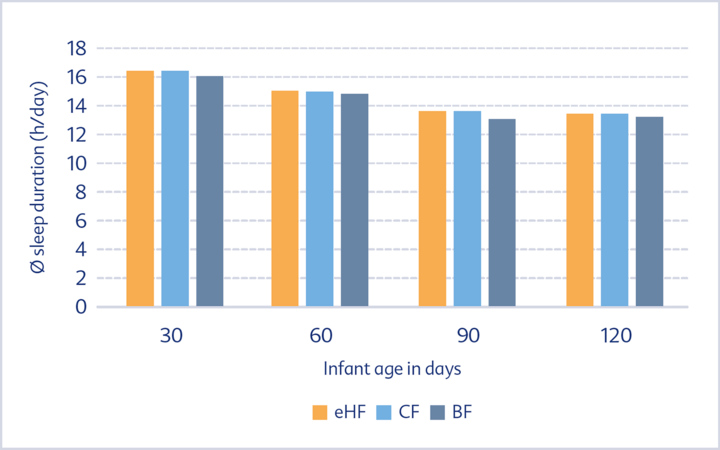
eHF, CF and BF groups during the first four months of life
The sleep behaviour of formula-fed infants was similar to that of breastfed infants. The average amount of sleep per day decreased with increasing infant age, whereas the amount of night sleep increased. In addition, the duration of uninterrupted night sleep increased in all participating groups and the time it took to fall asleep decreased over the observation period.
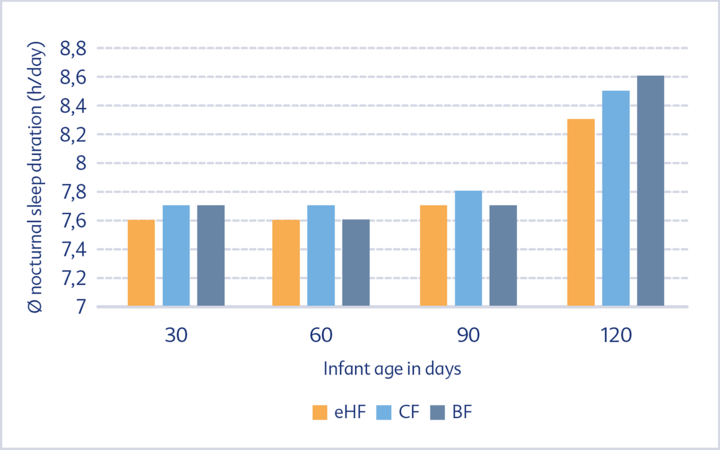
of the eHF, CF and BF groups during the first four months of life
HiPP ORGANIC COMBIOTIC® Products
All product information about our HiPP ORGANIC COMBIOTIC®.
HiPP information material




